Current status of cutaneous leishmaniasis in Australia
Background
Leishmaniasis is a well-documented disease of humans and animals worldwide. In humans, leishmaniasis is generally zoonotic with a number of different clinical manifestations ranging from single or multiple skin lesions (cutaneous leishmaniasis) to destruction of the mucosae, including the soft cartilage of the nasal septum (mucocutaneous leishmaniasis) or systemic infections of the liver and spleen (visceral leishmaniasis). The disease is caused by the single-celled, flagellate protozoan parasites Leishmania.
Over 20 species of Leishmania are known to cause leishmaniasis in humans and other animals.
The Leishmania parasite maintains a complex life cycle which involves a reservoir host (often asymptomatic), and a phlebotomine sand fly vector. In the mammalian reservoir host, Leishmania parasites exist as intracellular amastigotes within macrophages. However, in the sand fly gut and in vitro culture, they are extracellular, flagellate promastigotes.
Cutaneous leishmaniasis in macropods
Australia and Antarctica were thought to be the only continents free of Leishmania species and suitable phlebotomine sand fly vectors. However, in 2003, locally acquired Leishmania infections were identified in captive red kangaroos in the Darwin rural area of the Northern Territory. The Leishmania species causing this infection is now considered to be a unique Australian species.
Since then, clinical disease has been seen in other captive macropods, including several northern wallaroos, a black wallaroo and agile wallabies. In an attempt to implicate a reservoir, serological surveys of captive and free-range macropods were conducted in the Darwin rural area using an indirect enzyme-linked immunosorbent assay (ELISA) (unpublished study).

Figure 1: Ulcerated tail lesion of a red kangaroo (image adapted from Rose et al. 2004)
Depending on the locale, antibodies detected against a soluble Leishmania antigen of the Australian species were found in 5–20% (n = 157) of agile wallabies and 13–76% (n = 42) of antilopine wallaroos (M. antilopinus), indicating exposure or infection with an Australian Leishmania parasite. Tissue samples were also collected and tested for the presence of Australian Leishmania DNA by a specific real-time polymerase chain reaction (PCR). Of 17 Antilopine wallaroo ear snips tested, 53% were found to be positive for Leishmania DNA.
To elucidate the vector responsible for transmission of this newly identified species, field entomological investigations have been undertaken in the Northern Territory. A thorough field investigation was conducted in locations where Leishmania transmission was known to be occurring, and four species from the genus Sergentomyia were identified in the captive enclosures. Even though Sergentomyia spp. are considered a reptile-feeding sand fly, the collected females were screened for the Australian Leishmania DNA; none were found to be positive.
In addition, we found no mammal-feeding Phlebotomus spp. in our collections, leading us to conclude phlebotomine sand flies may not be involved in Leishmania transmission.
Subsequently, day-feeding midges observed feeding heavily on macropods in the monsoonal season were collected, revealing a potentially novel vector of Leishmania in Australia. The collections of day-feeding midges (Forcipomyia (Lasiohelea) spp.) contained three different species, including a new species designated Forcipomyia (Lasiohelea) sp. 1.
Specimens of this undescribed species showed a prevalence of Australian Leishmania infection of up to 15% (as indicated by real-time PCR). A prevalence of 6% was seen in F. (L.) peregrinator. Infections in these day-feeding midges have also been visualised by microscopy and promastigotes were cultured and identified in the laboratory as the same species infecting macropods.

Figure 2: Medial hock of a northern wallaroo depicting multiple smooth nodules and one ulcerated and encrusted lesion (Image: Cathy Shilton).
This is the first documented evidence of a potential vector other than phlebotomine sand flies anywhere in the world. To improve understanding of the Australian Leishmania lifecycle, continued surveillance and monitoring of its transmission is vital. To date, the Leishmania species has not been found in other states of Australia, although day-feeding midges are widely dispersed and are also found in New South Wales, Queensland and Western Australia.
Knowledge regarding these midges is scarce, and research into their biology and geographic distribution is important to improve the understanding of the potential risks of this disease. Now that we are aware of this parasite and its likely vector in Australia, veterinarians and wildlife carers need to be alert and on the lookout for infections.
Clinical disease
In macropods in the Northern Territory, the disease manifests itself as cutaneous lesions. The lesions are located distally in areas concentrated on the tail, inner forearms, hind legs and ears. Lesions on the limbs and tails can appear as focal to coalescing 0.5–2 cm diameter areas of thickened skin or raised variably encrusted or ulcerative pale nodules (Figures 1 and 2). On the ears, lesions primarily involve the distal margins of the pinnae, which are irregular in outline, mildly swollen and variably encrusted (Figure 3). The severity and number of lesions can vary among affected individuals. Naivety to infection and subclinical stress – related to an incompatibility with their captive conditions – may have contributed to the varying severity of disease seen in all of the macropod species described to date.
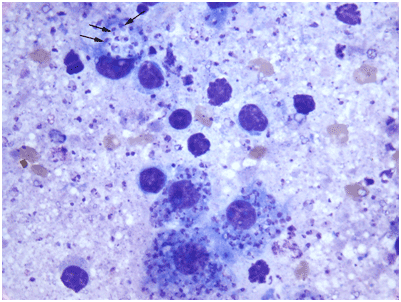
Figure 3: Agile wallaby ear; lesions on ears are located primarily at the distal margins, which are irregular in outline, mildly swollen and variably encrusted (Image: Jodie Low Choy)
Diagnosis
The gold standard for diagnosis is culture of the parasite from infected tissue. Small tissue samples (5 x 5 mm) or punch biopsy samples are suitable and should be aseptically collected from the lesion. Organisms are concentrated to the edge of the lesion, although this is not always the case; for macropod leishmaniasis, the organism is found throughout the nodular lesions. Samples collected for culture should be placed in a saline solution at room temperature. Ideally, culturing should be performed within 1 day of collection. Impression smears can be made before placing in saline, by lightly dabbing the tissue sample on a microscope slide. This can be stained either with Giemsa or Diff-Quik stain. Amastigotes (Figure 4) are easily seen by microscopic examination.
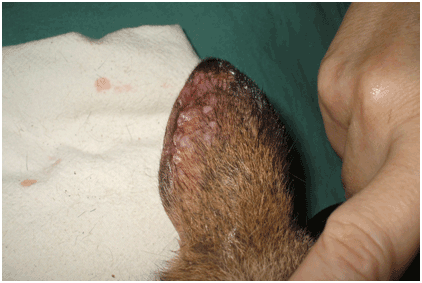
Figure 4: Impression smear from a northern wallaroo skin lesion depicting four infected macrophages among lymphocytes and cellular debris. Arrows point to three individual Leishmania amastigotes, each with round dark nucleus and single kidney kinetoplast. Diff-quik stain (Lab Aids Pty Ltd) (scale bar, 10 mm). (Image adapted from Dougall et al. 2009)).
Additional tissue samples can also be collected for histological examination (10% formalin) or for direct DNA detection via PCR (frozen dry or in ethanol). Parasites have also been detected in the cloaca, lymph node and spleens of infected northern wallaroos. If animals are euthansed, necropsy samples from these tissues should always be taken.
If logistically possible, blood should also be collected for serum to be used in Leishmania antibody detection assays.
Numerous methods for PCR detection and serology are described in the literature. However, the Australian Leishmania Laboratory (located at the Menzies School of Health Research in Darwin) will take samples from suspected cases of Australian Leishmania , isolated as above, for investigation by PCR, culture, histology or serology. The Australian Registry of Wildlife Health can undertake histology and cytology of specimens and the Australian Animal Health Laboratory can carry out serological assays for Leishmania .
Leishmaniasis is on Australia’s list of notifiable animal diseases. If you suspect or have confirmation of the disease, you must report it to your state or territory's department of primary industries or agriculture.
Relevance to Australia
The Australian Leishmania species is not known to infect humans, domestic animals or livestock in Australia, although the day-feeding midges bite various mammals such as humans, horses, ox, sheep, goats, rabbits and wallabies. The existence of a vector capable of transmitting Leishmania in the Northern Territory has raised the possibility that day-feeding midges may also have the capacity to carry and transmit exotic Leishmania species that might be introduced into Australia.
Cases of exotic leishmaniasis have been reported in Australia, particularly in returned travellers, refugees and occasionally in dogs imported from endemic regions. Current import conditions for dogs require serological testing for Leishmania infantum.
Preparedness for incursions of exotic diseases is of key importance to government, industry, producers and the Australian community. Should an introduced Leishmania species be found to be transmissible by day-feeding midges, the impact on human and animal health would be unknown and this highlights a need for continued awareness of, and surveillance for, Leishmania species in humans and animals.
Annette Dougall
Queensland Tropical Health Alliance
James Cook University
Deborah Holt
Menzies School of Health Research
Charles Darwin University
Further reading
Rose K, Curtis J, Baldwin T et al. Cutaneous leishmaniasis in red kangaroos: isolation and characterisation of the causative organisms. Int J Parasitol 2004;34:655–664.
Dougall A, Shilton C, Low Choy J, Alexander B, Walton S. New reports of Australian cutaneous leishmaniasis in Northern Australian macropods. Epidemiol Infect 2009;137:1516–1520.
Dougall AM, Alexander B, Holt DC et al. Evidence incriminating midges (Diptera: Ceratopogonidae) as potential vectors of Leishmania in Australia. Int J Parasitol 2011;41:571–579.
Debenham ML. Australasian Species of the Blood-feeding Forcipomyia Subgenera, Lasiohelea and Dacnoforcipomyia (Diptera: Ceratopogonidae). Australian Journal of Zoology Supplementary Series 1983;31:1–61.
Figure 1: Ulcerated tail lesion of a red kangaroo (image adapted from Rose et al. 2004)
Figure 2: Medial hock of a northern wallaroo depicting multiple smooth nodules and one ulcerated and encrusted lesion (Image: Cathy Shilton).
Figure 3: Agile wallaby ear; lesions on ears are located primarily at the distal margins, which are irregular in outline, mildly swollen and variably encrusted (Image: Jodie Low Choy)
Figure 4: Impression smear from a northern wallaroo skin lesion depicting four infected macrophages among lymphocytes and cellular debris. Arrows point to three individual Leishmania amastigotes, each with round dark nucleus and single kidney kinetoplast. Diff-quik stain (Lab Aids Pty Ltd) (scale bar, 10 mm). (Image adapted from Dougall et al. 2009).
