Peste des Petits Ruminants
The situation in South Asia
Since Peste des Petits Ruminants (PPR) was reported in the Middle East in 1980s, there has been growing concern over the spread into Central and Southern Asia.1 PPR has caused significant economic loss in many parts of Africa and Asia that contain high densities of small ruminant populations. According to a 2009 United Nation’s Food and Agricultural Organisation (FAO) report, the proportion of the global small ruminant population at risk from PPR is estimated to be 62.5%.
PPR is a highly contagious viral disease of sheep and goats. On 25 July 2007, China officially reported to the World Organisation of Animal Health (OIE) its first case of PPR in the Ngari region of western Tibet. The outbreak resulted in 292 small ruminant deaths and 2494 goats being destroyed, but was resolved in February 2008. On 11 July 2008, the Chinese Chief Veterinary Officer confirmed and reported a second outbreak within the Tibet province. No mortalities occurred, and this outbreak was resolved in December 2008.
The China Animal Health and Epidemiology Centre in 2009 reported that the epidemiological and serological evidence of PPR in Tibet suggested that it had existed for several years without detection, most likely due to animal health workers being unfamiliar with the disease. Further data suggested cross border transmission of PPR into Tibet. PPR has been recognised since the 1990’s in countries bordering south-western China, including India, Nepal, Bangladesh, Pakistan and Afghanistan.
The uncontrolled movement of small ruminant herds between countries has been a key factor in the spread of PPR in the south west Asia region over the past 10 years. Investigations of outbreaks in Africa emphasise that almost all outbreaks have been traced back to stock movements such as migration or introduction of new animals.
In many Asian countries, the terrain and extent of small ruminant trade makes monitoring and restricting animal movements difficult and therefore increases the risk of the disease crossing borders and establishing in countries previously free of PPR.
Epidemiology of the disease
The natural hosts are goats and sheep. The disease has also been described in wildlife such as gazelles and ibex, but these are not significant to PPR epidemiology.1 Subclinical infection of cattle and pigs can occur, but these species do not readily transmit the disease. Currently, there are no known health risks or human infections caused by PPR virus.
There are four PPR virus lineages (1-4) that are distinguished by their nucleic acid sequences and geographic location. Lineage 1 and 2 predominately occurs in parts of Africa. Lineage 3 has been reported from parts of Africa, the Middle East and southern India, but no further isolations were identified since the one reported in India in 1992.2 The virus in the 2007 China outbreak was designated as lineage 4, which is endemic in the Middle East and Indian subcontinent.
Sheep and goats have different susceptibilities to the four lineages. Age is also an important factor with animals less then 2 years of age being more severely affected than adults and unweaned young. The beginning of the rainy season and cold, dry periods have been associated with an increase in outbreak frequencies.
PPR has two clinical forms, peracute and acute. Peracute cases commonly occur more in goats. A high fever is followed by depression and death, and mortalities are normally very high. In acute forms, the incubation period is between two to six days, followed by high fevers, mucopurulent ocular and nasal discharges, pneumonia, necrosis and inflammation of oral mucous membranes and severe diarrhoea. Coughing, difficulty in breathing and small nodular lesions around the lips and muzzle may be observed. Morbidity rates in naïve populations can be 90 to100%, with mortalities reaching 50 to 90%.
Geographical distribution
The first case of PPR was identified and described in 1942 in Ivory Coast, Africa before it spread to West Africa. In the early 1980s, PPR then spread east into Sudan followed by India in 1987, where epidemics occurred for several years without spread. The Arabian Peninsula, Middle East and the remaining part of the Indian sub-continent were later swept by an epidemic in the mid-1990s. Currently, PPR is identified in almost all countries of the Middle East; in Central and South Asia; and, in Africa between the Sahara and the equator. Morocco and Tanzania recorded their first case of PPR in 2008 and 2009 respectively.
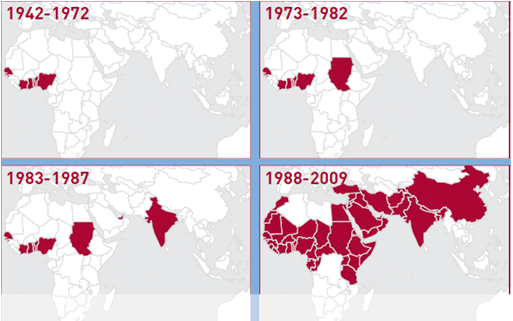
Figure 1: Map of PPR geographical distribution (FAO 2009)
How is PPR transmitted?
The apparent increase in PPR cases in recent decades is most likely associated with a combination of increased awareness, improved and more available diagnostic tools and a change in the nature of the virus. Developments in transport, tourism and migration patterns of wild animals susceptible to PPR may also be contributory factors. Outbreaks have also been associated with cultural festivals and the associated movements of large numbers of small ruminants.
Infected sheep and goats can shed the virus in all secretions and excretions, including tears, nasal discharge, faeces and urine, and transmission is predominantly by aerosol inhalation or direct contact, such as licking. The virus has poor survival outside the host, but other sources include contaminated water troughs, bedding and feed.
How is PPR diagnosed?
A combination of the following history and clinical signs would warrant the suspicion of PPR:
- acute onset of high fevers and diarrhoea in goats and sheep
- high morbidity and mortality rates in animals of all ages
- ocular and nasal discharges
- pneumonia and mouth lesions.
A tentative diagnosis based on clinical signs is possible but difficult, as the diagnosis can be easily confused with other diseases such as foot and mouth disease, rinderpest, contagious bovine pleuropneumonia, pasteurellosis and bluetongue. Laboratory diagnosis is required for a definitive diagnosis. The prescribed test for trade is the virus neutralisation test. Because PPR virus is closely related to rinderpest virus, this test is important in differentiating rinderpest and PPR antibodies. The Australian Animal Health Laboratory (AAHL) currently offers PCR, virus isolation, electron microscopy, sequence analysis and serum neutralisation tests.3
In suspected cases of PPR, appropriate collection and transport of specimens is important. The virus is present for approximately 10 days after the onset of fever and can be isolated during the acute stage of the disease when clinical signs are still apparent from conjunctiva, nasal, oral and rectal mucosa swabs; clotted and whole blood (in EDTA anticoagulant); lymph node or spleen biopsies.
Samples of spleen and lymph nodes from freshly slaughtered animals or fresh carcasses should be collected for virus isolation. For histopathology samples of tongue, spleen, lung, lymph nodes, affected segments of the intestinal tract are required.2,3 Unpreserved tissue, blood or swab specimens must be chilled with water ice or frozen packs. Specimens should be sent to state diagnostic laboratories for forwarding to AAHL.
How can PPR be managed?
Currently, there is no specific treatment for animals with PPR infection.
Previously, a rinderpest tissue culture vaccine was used for PPR control. This has been replaced by a homologous attenuated PPRV vaccine. Currently there is no registered or permitted vaccine for PPR in Australia. There is no method of distinguishing vaccinated and infected animals, a situation which can interfere with control and eradication measures.
The AUSVETPLAN strategy3 for an epidemic outbreak of PPR would involve stamping out and the use of other strategies such as:
- early recognition and laboratory confirmation of all cases
- strict quarantine and restriction on animal movements
- disposal and destruction of all infected animals and products
- decontamination of all in contact premises
- tracing and surveillance
- declaration of disease and disease free areas.
How could PPR enter Australia?
Australia has not reported any cases of PPR to date.
Although increased recognition of PPR is contributing to the observed increase in prevalence, the virus is spreading. According to FAO,1 the disease is known to be extending in an eastward direction through western and southern Asia. This reflects the major challenges in the control of PPR within the southern Asian continent, where there is a lack of experience with the disease and control of animal movements in nomadic animal production systems.
In Australia, a potential outbreak of PPR would cause serious economic loss in the Australian sheep and goat industry and local communities. Based on Australia’s geographical isolation, the main routes of viral introduction would be by importation of PPR infected sheep or goats and contaminated dairy products such as milk and semen or embryos.2 Australia currently only imports live sheep and goats from New Zealand, a PPR-free country. However, Australia does import sheep and goat semen from Canada, New Zealand, the European Union and the United States of America under strict biosecurity and specific conditions. Sheep and goat embryos are also imported from those countries and the Republic of South Africa, subject to specific conditions. The illegal introduction of sheep or goat dairy and meat products, semen and embryos poses the main risk for PPR introduction into Australia.3
Warning for Australian veterinarians
The risk of PPR introduction is regarded as remote with Australia’s current quarantine and inspection services regime and the restrictions placed on importation of live animals, genetic material and animal products. However the evolving situation in Asia should serve as a reminder to Australian veterinarians that being current and familiar with exotic disease information will assist in maximising Australia’s chances of early recognition and laboratory confirmation of a potential epidemic outbreak.
In PPR, early recognition and warning is the key to an early reaction towards control, containment and elimination 4.
EMERGENCY ANIMAL DISEASE WATCH HOTLINE — 1800 675 888
1. Food and Agriculture Organisation of the United Nations (FAO). Peste des Petit Ruminants (PPR) A challenge for small ruminant production. 2009http://www.fao.org/docs/eims/upload/258701/ak065e00.pdf . Accessed 3 May 2010
2. World Organisation for Animal Health (OIE). Technical Disease cards: Peste des Petits Ruminants 2009. http://www.oie.int/eng/maladies/Technical%20disease%20cards/PESTE%20DES…. Accessed 2 May 2010
3. AUSVETPLAN, Animal Health Australia. Disease Strategy – Peste des petits ruminants 2009. http://www.animalhealthaustralia.com.au/fms/Animal%20Health%20Australia… Accessed 1 May 2010
4. Food and Agricultural Organisation of the United Nations (FAO) Animal Health Manual. Recognising Peste des Petits Ruminants: A field manual. 1999 ftp://ftp.fao.org/docrep/FAO/003/X1703E/X1703E00.PDF Accessed: 4 May 2010.
