Authors: Alice Kermond, Jenny Baird and Sally Thomson (Department of Agriculture, Water, and the Environment)
African Horse Sickness
African horse sickness virus (AHSV) is a highly pathogenic arbovirus with the potential to cause severe, often fatal, circulatory, and respiratory disease in horses. It is a nationally notifiable disease that is exotic to Australia. Historically the spread of African horse sickness (AHS) has been limited to Africa with occasional outbreaks in Europe, making the recent incursions of AHS in Malaysia and Thailand of particular significance. Horses are most severely affected, however all species within the Equus genus are susceptible, including donkeys, mules, and zebra. Zebra are considered the natural hosts and act as a reservoir by having an extended period of viraemia whilst exhibiting few or no clinical signs. Disease with mortality has been observed in dogs following ingestion of infected horse meat. Seroconversion has also been observed in camels, however there is no evidence to suggest camels contribute to the persistence and spread of the virus.
Aetiology
AHSV is a double stranded RNA virus belonging to the family Reoviridae, genus Orbivirus. There are nine serotypes and it is morphologically similar to Bluetongue virus. Prior to incursion of serotype 1 in Thailand, outbreaks have been associated with serotypes 4 and 9.
AHSV can survive a few hours in carcasses but is readily inactivated once the carcass pH falls below 6.0 as part of the normal decomposition process.
Distribution/geographic spread
The distribution of AHSV is dependent on the presence of a suitable vector and equid host. In Africa the principal vector is the biting midge Culicoides imicola.
AHS is endemic across central Africa with regular spill-over into southern Africa. In the past century there have been outbreaks in Africa, the Middle East, Asia, and Europe. In March 2020 Thailand reported its first case of AHS which is suspected to be due to the importation of infected zebras. The most recent incursion of AHS occurred in Malaysia. The mode of entry and serotype is currently unknown, and investigations are ongoing. More information is available on the OIE website (OIE 2020).
Transmission
AHS is transmitted by biting midges of the Culicoides genus. The Culicoides midge ingests blood containing the virus from an infected horse. The virus then migrates to the salivary gland of the midge before being transmitted when the midge next feeds on another horse.
C. imicola is the only confirmed vector in Africa however C. bolitinos is strongly suspected to act as a competent vector. Although C. imicola and C. bolitinos are exotic to Australia there are several other Culicoides species endemic to Australia which could potentially serve as AHSV vectors. Of concern is C. brevitarsis which is a vector of Bluetongue virus and Akabane virus.
Although AHSV has been demonstrated in equine semen and embryos, the significance of this is unclear. There are no records of transmission of disease by reproductive material.
Clinical disease
Following an incubation period of 3-14 days, most infected zebras and some donkeys remain asymptomatic. Naïve horses almost always develop severe disease, displaying a range of cardiovascular and/or respiratory signs.
There are four recognised clinical presentations of AHS
- Horse sickness fever: occurs in horses previously exposed to the infecting serotype, or in naturally resistant equids such as the African donkey or zebra. Clinical signs include mild fever, malaise, and mild supraorbital fossa oedema. Mortalities are uncommon.
- The cardiac form: horses present with a fever of 39-41℃ and subcutaneous oedema affecting the supraorbital fossa, neck, chest, and shoulders. The mortality rate is 50%.
- The mixed form: the most common presentation. It is a combination of cardiac and pulmonary clinical signs including fever, oedema, depression, and moderate dyspnoea with a mortality rate of 70-80%.
- The pulmonary form: horses are markedly depressed, have a fever >40℃ and are severely dyspnoeic. The mortality rate is 95% with death occurring within 7 days of displaying clinical signs.
Viraemia lasts 4-8 days (maximum of 21) in horses, 4 weeks in donkeys and 6 weeks in zebra. This prolonged period of viraemia in donkeys and zebra contributes to their success as maintenance hosts.
In rare cases of recovery, horses develop antibodies against the infecting serotype providing a degree of immunity to reinfection with that serotype.
Diagnosis
AHS should be on the differential list for horses presenting with fever along with severe respiratory and cardiovascular symptoms, as well as sudden death. Laboratory testing is required for definitive diagnosis as AHS can appear similar to other diseases including Hendra, piroplasmosis, and equine viral arteritis.
Samples to collect for laboratory testing include:
- whole blood – in EDTA tubes
- serum – in plain tubes
- fresh tissue including spleen, lungs, and lymph nodes
Blood should be collected from live, clinically affected animals, ideally early in the development of clinical signs. Fresh tissues should be collected from recently dead animals. Blood and fresh tissue samples should be chilled and transported at 4°C with frozen gel packs. Samples should not be frozen as this reduces the sensitivity of virus isolation and molecular diagnostic tests.
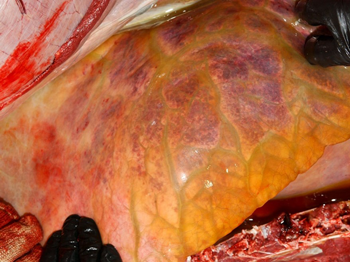
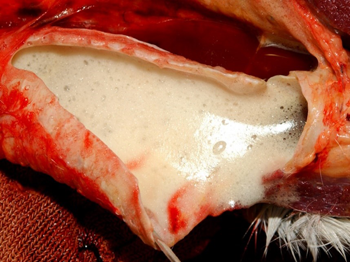
The post-mortem lesions vary depending on the disease form. In the pulmonary form, lesions include interlobular oedema of the lungs (Figure 1), hydrothorax, hydropericardium, frothy discharge within the trachea (Figure 2), oedema of the lymph nodes, and petechial haemorrhage on serosal surfaces. Occasionally congestion in the renal cortex and oedematous infiltration around the aorta and trachea may be seen.
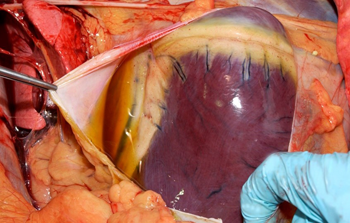
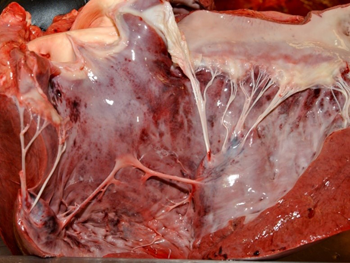
Common lesions in the cardiac form include hydropericardium (Figure 3); petechial to ecchymotic haemorrhages of the epi- and endocardium (Figure 4); yellow gelatinous infiltrate of the fascia of the head, neck, and shoulders; and occasionally petechial haemorrhages of the gastrointestinal serosa.
Post-mortem findings for the mixed form are a combination of the pulmonary and cardiac lesions.
(Source: United States Department of Agriculture, Plum Island Animal Disease Center.)
(Source: United States Department of Agriculture, Plum Island Animal Disease Center.)
(Source: United States Department of Agriculture, Plum Island Animal Disease Center.)
(Source: United States Department of Agriculture, Plum Island Animal Disease Center.)
Risk to Australia of introduction of AHS
Introduction of AHS virus to Australia could occur through an infected equid or a live infected vector.
While importation of a viraemic equine host is possible, Australia’s import conditions significantly reduce this risk by prohibiting the entry of equids and equid genetic material from countries with AHS.
Windborne spread of vectors is possible as Culicoides can travel up to 700km over water in windy conditions. Research is underway in northern Australia to identify what Culicoides species feed on horses and whether they are competent vectors.
Control
If AHS were to occur in Australia the policy as described in AUSVETPLAN is to eradicate the disease through activities such as movement controls, insect vector control, surveillance, appropriate carcass disposal, and a public awareness campaign.
Methods of vector control include removing suitable breeding areas such as stagnant water and damp shady areas, cutting grass short, strategic use of insect repellents and insecticides, and stabling horses in midge proof enclosures. C brevitarsis also feeds on cattle and can survive in cattle dung allowing them to be less dependent on outside water sources than many Culicoides. Therefore, separating horses from cattle may reduce the risk of exposure to C brevitaris.
For further details on Australia’s response policy, see the AUSVETPLAN AHS manual located on Animal Health Australia’s website: https://www.animalhealthaustralia.com.au/our-publications/ausvetplan-manuals-and-documents/. This manual is in the process of being updated, with the revised version expected to be completed in early 2021.
Keeping Australia AHS free
Australia has never had an outbreak of AHS and maintains its AHS-free status through biosecurity policies and border controls. Australia has strict import conditions for live equids, genetic material, vaccines, and other commodities that may be contaminated with AHS virus or Culicoides.
Australia continues to monitor the global spread of AHS to ensure these biosecurity policies and import controls are appropriate. The Northern Australian Quarantine Strategy (NAQS) conducts surveillance of feral horses and donkeys, monitoring for any suspect deaths and early warning signs. NAQS also contributes to the National Arbovirus Monitoring Program for Culicoides vectors of Bluetongue virus, another key feature in Australia’s surveillance for potential AHS vectors.
How Australian veterinarians can help
Although the risk of AHS entering Australia is low, the global distribution is changing, and it is important that Australian veterinarians maintain current knowledge and remain alert to exotic disease risks such as AHS. Prevention and early detection and laboratory confirmation are vital and veterinarians have an important role to play to ensure a rapid and effective response.
Veterinarians should report any unusual or suspect cases of emergency animal diseases to a state/territory government veterinarian or by calling the Emergency Animal Disease watch hotline (1800 675 888).
References
Bellis,G, Thepparat, A, Wardhana, A, Le, L, Duan, Y & Jiahui, L 2020, Potential vectors of African horse sickness in Asia OIE Webinar
Hendrickx, G, Gilbert, M, Staubach, C, Elbers, A, Mintiens, K, Gerbier, G & Ducheyne, E 2008 A wind density model to quantify the airborne spread of Culicoides species during north-western Europe Bluetongue epidemic, 2006 Preventative Veterinary Medicine 15;87(1-2)162-81
Kolk, JH van der. & Veldhuis Kroeze, EJB 2013, Infectious diseases of the horse: diagnosis, pathology, management, and public health, Manson Publishing Ltd, London.
OIE 2013, African Horse Sickness Technical Disease Card (pdf 221kb), World Organisation for Animal Health, Paris, accessed 30 June 2020.
OIE 2020, African Horse Sickness, World Organisation for Animal Health, Paris, accessed 14 January 2021.
