The following is provided as published in the Australian Veterinary Journal, Volume 95, Number 5, May 2017. The information in the disease bulletin was correct at the time of publication.
Authors: Corissa Miller & Anna Kabaila (Australian Department of Agriculture and Water and the Environment).
Seneca Virus A: An emerging disease
SVA is a non-enveloped single-stranded RNA virus belonging to the family Picornaviridae, of which swine vesicular disease (SVD) and foot-and-mouth disease (FMD) are also members. The virus was discovered in 2002 as a cell culture contaminant, but archived samples suggest it has been present in the United States (US) since the 1980s. The first animals to be clinically affected by SVA were identified at a US abattoir in 2007. The virus has since been confirmed as the causative agent in outbreaks of vesicular disease in the US, Canada, Brazil and China. SVA has also been associated with a syndrome known as epidemic transient neonatal losses (ETNL) in piglets.1 Globally, the incidence of SVA appears to be on the increase, with a surge in the number of cases reported in the US since July 2015. SVA has never been detected in Australia. However, it is important that Australian veterinarians remain alert to emerging disease risks, such as SVA, to ensure rapid recognition and response to a potential outbreak.
What clinical signs would you expect to see?
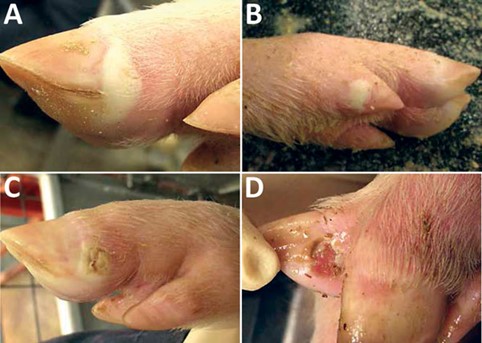
The clinical signs of SVA are indistinguishable from those caused by other exotic vesicular diseases of pigs, such as FMD, SVD, vesicular stomatitis (VS) and vesicular exanthema of swine (VES). Clinical signs in piglets can also resemble porcine epidemic diarrhoea (PED) and transmissible gastroenteritis (TGE). SVA is therefore an important differential diagnosis for a number of emergency animal diseases (EADs).
SVA in grower and finisher pigs is characterised by vesicular lesions on the snout, feet and limbs. Lameness is commonly observed and gross lesions include vesicles and ulcerative lesions on distal limbs, especially around the coronary bands; crusting and sloughing of the hoof; and vesicles and ulcers of the oral mucosa, snout and nares. The pathogenicity of SVA remains unclear and limited data are currently available on associated morbidity and mortality in adult pigs. Reports of recent outbreaks in the US suggest a high morbidity of 80 to 90 per cent, with low mortality in finisher pigs and an average recovery time of 30 days.
Clinical signs observed in piglets infected with SVA include diarrhoea, weakness, lethargy, excessive salivation, cutaneous hyperaemia and neurological signs. Although clinical signs resolve in surviving piglets after seven to 10 days, a two to 10 per cent increase in post-weaning mortality has been reported in infected herds.
Disease dynamics – what do we know?
There is little published information on the epidemiology or transmission of SVA in pigs, including transmission routes, whether a carrier state exists, the role of non-porcine hosts and virus survival. A brief summary of recent studies is presented below.
Transmission
A range of transmission pathways should be considered until further information becomes available, including ingestion or inhalation of secretions, excretions or products, exposure to fomites and mechanical transmission by vectors such as biting flies. Experimentally, clinical infection with SVA was achieved by the intranasal inoculation of pigs2. Studies have found that the viraemic period is short, lasting between three and 10 days post-inoculation. Viral shedding occurs from one to 28 days post-inoculation, in oral and nasal secretions and in faeces3.
Survivability
As SVA is a member of the Picornaviradae family it is considered to be a relatively robust and environmentally stable virus. Investigations following previous outbreaks have demonstrated the presence of viable SVA in various environmental samples from affected farms, including farm tools, building surfaces and equipment. The virus was also detected in multiple mouse samples (faeces and intestinal tissue), demonstrating that mice can carry and possibly shed the virus4.
Affected tissues
Samples from field studies have detected virus in oral fluids for at least four weeks from the start of an outbreak, and beyond resolution of vesicular lesions. High viral loads have been detected in a wide range of tissues, suggesting SVA can replicate in multiple organs.
Viral ecology
The RNA genome of SVA contains similarities to viruses of the family Flaviviridae (such as classical swine fever), suggesting the possibility of recombination events between the genomes of Picornaviridae and Flaviviridae5. It has been hypothesised that genetic exchange between members of Picornaviridae and Flaviviridae during persistent co-infection in pigs may have resulted in the development of SVA.
How might SVA impact Australia?
Because SVA is clinically indistinguishable from a number of EADs, detection of vesicular disease associated with SVA may result in costly quarantine and movement restrictions during the initial phase of a disease investigation. This may also cause temporary disruptions to trade and suspend or limit access to some export markets until EADs such as FMD, SVD, VE and VS are ruled out.
If ETNL associated with SVA was to occur in Australia, transient neonatal disease with potentially low mortalities may not be investigated initially. If an outbreak of SVA were not immediately recognised, SVA could become endemic within a herd before being identified, with direct impacts on herd production.
Furthermore, the establishment of SVA in Australia could delay detection and response to EADs.
How can Australian veterinarians help?
The monitoring of global disease outbreaks such as SVA is necessary to maintain a strong biosecurity framework and protect Australia’s globally competitive and sustainable livestock industries.
Live pigs and their genetic material are not permitted import into Australia. Pig meat and pig meat products are required to be cooked or cured and specific tissues are removed to manage the biosecurity risks. Thus under Australia’s current biosecurity protocols and importation restrictions, the risk of SVA introduction is likely to be low. However, the level of risk posed by SVA to Australia is difficult to quantify until more information on the virus, transmission pathways, the global distribution and other epidemiological data are understood. Subclinical or mild disease is difficult to detect and may result in delayed responses and more significant outbreak impacts.
It is therefore important that Australian veterinarians maintain current knowledge and remain alert to emerging and emergency animal disease risks, to ensure rapid recognition and response to a potential outbreak. Early detection of SVA is key to effective disease control.
If you suspect an exotic disease, please contact the Emergency Animal Disease Watch Hotline on 1800 675 888 for advice and assistance.
Further reading
1 DEFRA 2016, ‘Emerging pig disease associated with Seneca Valley virus in the Americas’, Department for Environment, Food and Rural Affairs, Animal and Plant Health Agency, Veterinary and Science Policy Advice – International Disease Monitoring.
2 Montiel N, Buckley A, Guo B, Kulshreshtha V, VanGeelan A, Yoon K, Lager K 2016, ‘Vesicular disease in 9-week-old pigs experimentally infected with Senecavirus A’, Emerging Infectious Diseases, vol 22, no. 7, pp. 1246-1248.
3 Joshi L, Fernandes M, Clement T, Lawson S, Pillatzki A, Resende T, Vannucci F, Kutish G, Nelson E, Diel D 2016, ‘Pathogenesis of Senecavirus A infection in finisher pigs’, Journal of General Virology, vol 97, no. 12, pp. 3267-3279.
4 Joshi L, Mohr A, Clement T, Hain K, Myers B, Yaros J, Nelson E, Christopher-Hennings J, Gava D, Schaefer R, Caron L, Dee S, Diel D 2016, ‘Detection of the emerging picornavirus Senecavirus A in pigs, mice and houseflies’, Journal of Clinical Microbiology, vol 54, no.6, pp. 1536-1545.
5 Willcocks M, Locker N, Gomwalk Z, Royall E, Bakhshesh M, Belsham G 2011, ‘Structural features of the Seneca Valley virus internal ribosome entry site (IRES) element: a picornavirus with a pestivirus-like IRES’, Journal of Virology, vol 85, no.9, pp. 4452-4461.
