Issue Date: 6 July 2016
Contact Officers:
Baden Pearse
07 3246 8764
Sam Allan
07 3246 8606
Date of Effect: Immediate
Date of Review: 1 July 2018
Distribution Categories
Central and regional office
Departmental on-plant officer(s)
Managers, export meat establishments
Purpose
This Notice replaces Meat Notice 2013/05 and clarifies the new regulatory procedure to inspect finished USA-eligible, sheep, lamb and goat product (carcases or carton meat product) immediately prior to or after packaging. The aim is to ascertain if the product is free of defects that could result in a Port of Entry (POE) rejection in the USA. If such defects are found, the establishment will be subjected to the same regulatory action that would follow a POE rejection. This Notice also provides occupiers with the option to designate some of their production as being non-USA eligible providing they implement appropriate identification and inventory controls.
This Notice removes bobby calves from the scope. It now only applies to sheep, lamb and goat products.
This Notice must be implemented no later than the date listed above. If the company has not made approved changes to their Approved Arrangement by that date, all product will be assumed to be USA eligible and hence be included in the daily Department of Agriculture and Water Resources product verification.
Scope
The Notice applies to the inspection of finished, sheep, lamb, and goat product (carcases or carton meat product) in boning rooms (integrated and independent), bagging chillers and cold stores which the establishment has designated to the On-Plant Veterinarian (OPV)/Food Safety Meat Assessor (FSMA) to be USA eligible. The lines of eligible product may change from day to day as the occupier decides the appropriate markets for individual lots.
Definitions
The following table defines terms used in this notice.
| Term | Definition |
|---|---|
| Corrective Action Plan | A comprehensive documented plan that ensures deficient activities are addressed in a sustainable manner and is agreed between the occupier and the department. The plan must contain an investigation of cause, consideration of corrective and preventative measures, and implementation of corrective action, monitoring, assessment and verification. |
| Daily product hygiene verification | Inspection of USA-eligible finished product as detailed in this Notice. |
| Product selection and inspection site | The product will be selected from the most convenient site in the production chain where the occupier has finished any trimming on the product but before the product is packaged so as to reduce inspection times and damage to packaging materials. It is permissible, but not preferable, to inspect packaged carcases/cartons. The selected product shall be examined at a site with adequate light (600 lux minimum). If the Carton Meat Assessment area is not being utilised by the company, the department may use that area for inspecting boning room product, in consultation with the management. Alternatively, the product can be inspected at any suitable area within the boning room, chiller or meat inspection area. |
| USA Port of Entry defects | Defects, on meat or carcases, identified at Port of Entry inspection and deemed to be reason for rejection. These include faeces/ingesta/milk, off condition, other chemical or physical hazards, other harmful material or conditions, pathological and parasitic lesions. |
Background
In 2012, the Critical Incident Response (CIR) audit was implemented. An official notification of a POE rejection in some markets will trigger a CIR audit at one of three levels, based on the number of rejections an establishment experienced within a moving 12 month window.
While this regulatory response was implemented as part of the department’s response to market expectations, due to further POE rejections for faecal/ingesta contamination of sheep and goat product, it is necessary to further enhance the regulatory approach to reduce the risk of market failure.
Under Australian regulation, the registered occupier (processor) is responsible for the food safety, hygiene and integrity of their products. The department, through sampling, verifies that the occupier is meeting their legal obligations and on that basis provides certification.
Methods
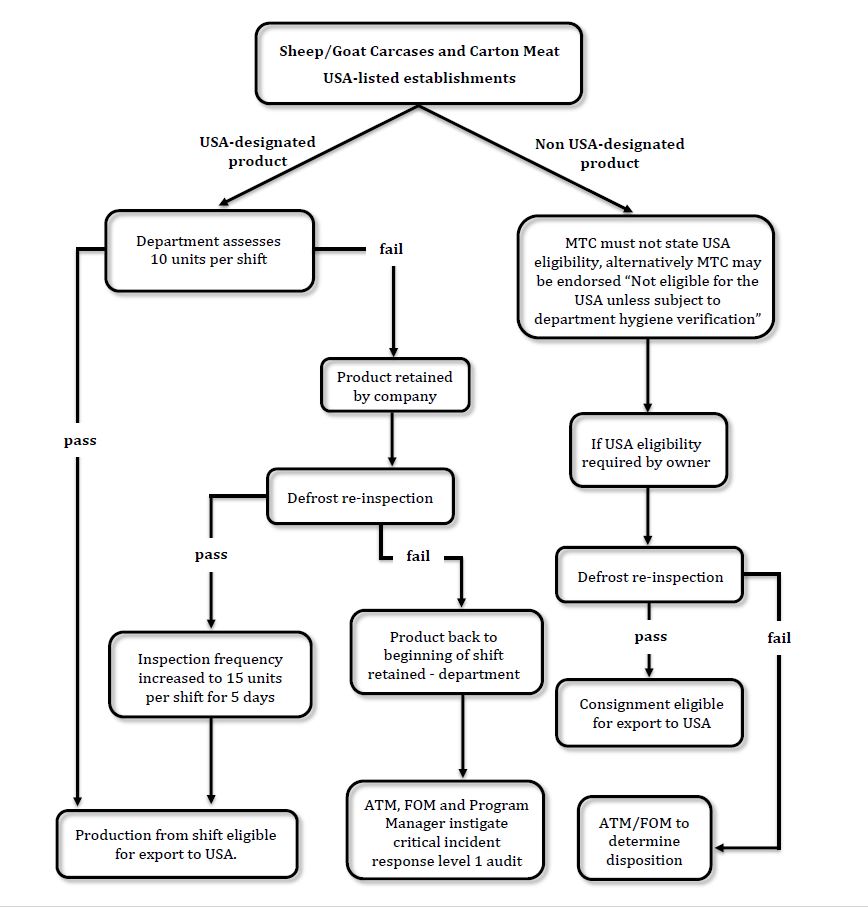
The OPV/FSMA will inspect 10 units of finished, USA-eligible sheep, lamb and goat product per shift. A unit is a carcase (if exported whole) or 5.5 kg of carton of meat. These 10 units may consist of a mixture of bagged carcases or carton meat product. The department will decide each day, after being informed by management which product for the shift is US eligible, what mix of carcase and carton product will be selected, and what product lines will be inspected. The 10 units do not have to be inspected at the one time and preferably will be inspected on more than one occasion during the shift.
The OPV/FSMA will choose what they think is the highest risk (of USA rejection) lines from the list of US eligible lines. The highest risk product is that with external surfaces.
When the OPV/FSMA arrives in the boning room/chiller they must consult with the designated management representative (usually the supervisor or QA officer) who will inform them what product lines are US eligible. The OPV/FSMA will decide which products from the eligible list of products will be inspected and how many units of each, making up a total of 10 units per shift. Accompanied by the designated management representative, the OPV/FSMA will select the carton of finished product or the required number of pieces of meat, immediately prior to packaging to inspect.
It is important that the correct amount of product is selected. A whole carcase or 5.5 kg of meat from each carton is examined. Once that unit has been inspected, the next unit is selected and inspected until the required number of units has been inspected.
If there is no USA eligible production on a particular shift, then no action under this Notice is required for that shift.
During the inspection, the defects of interest are those that may result in a Port of Entry rejection in the USA. These are referred to as “Port of Entry Defects” in the Notice and include faeces and ingesta, pathology, parasitic lesions and some other hide-related defects that may be a mixture of faeces and dirt, and are sufficiently numerous to be a risk of POE rejection. This procedure replaces the superseded Order 285 in the Meat Hygiene Assessment (MHA) program.
Record findings in Form 1 (Appendix 1).
Examples of POE defects (Directive 9900.2)
Defects that have, or appear to have, the appearance of originating from the gut such as faeces, ingesta, patch of hide dirt/wool dust, clump of wool mixed with greenish organic matter (excluding singed bristle), or of discoloured meat surface that has a similar colour to faeces/ingesta.
For goats, USA POE rejections have occurred for excessive hair contamination. As the FSIS do not stipulate the quantity of hair that would “adversely affect the suitability of the product”, as a general guide, more than 20 loose strands or more than 3 clusters would be excessive if identified in more than one sample unit.
For Cysticercus ovis (sheep measles) lesions in sheep, as it is not a food safety issue, there is a tolerance of one lesion in the 10 units. As parasitic lesions are listed as a category in POE defects, more than one lesion in the 10 units should be classified as a POE defect.
Examples of defects not likely to be POE defects
Wool, loose hair, specks, bruises, grass seeds – providing they are not sufficient to adversely affect the suitability of the product.
For skin-on goat, shaved bristles, providing they are short, should not be classified as a POE defect. Similarly, for pigmented skin.
Flowchart indicating the various pathways for sheep, lamb and goat product at USA-listed establishments
Instructions for completing Form 1
The columns, numbered 1 to 10, are for the 10 units of USA-eligible sheep, lamb and goat product to be examined by the OPV/FSMA each shift. A unit is a whole carcase or 5.5kg meat from carton product. Product should be examined immediately prior to bagging or packaging, and after the company has finished trimming the product. All USA eligible products are in scope for examination including vacuum product. The occupier will inform the OPV/FSMA on a daily basis what products are USA eligible and should accompany the OPV/FSMA during the inspection, where possible.
In the Species row, record whether the product is from sheep (S), lamb (L) or goat (G). In the Product Description row, record the type of product examined eg. racks.
There are four types of Port of Entry Defects:
- Faeces/Ingesta, Hide dirt, Smears, Green Discolouration – anything that has the appearance of having come from the gastro-intestinal tract or from faeces off the hide which at Port of Entry inspection may cause a rejection
- Physical hazards – any amount of foreign or hazardous material capable of causing injury or illness eg metal, glass, hard plastic, wood or unidentifiable material of size rendering the product unwholesome
- Other harmful material or conditions – any amount of the following that renders the product unwholesome: large insects associated with unsanitary conditions, evidence of rodent activity. Defects of a number and size seriously affecting product usability for further processing
- Pathological and parasitic lesions – single or multiple lesions that seriously affect product usability and renders the product unwholesome.
Procedures following a POE defect finding
- The offending product line packed earlier in the shift shall be retained by the occupier. The retention is unaffected if that line contains more than one slaughter date. While the defects emanated from the slaughter floor, the last critical control point that failed occurred on the packing date and so it is the product line that is packed that shift that is retained for reinspection, regardless of slaughter date.
- Inform the Area Technical Manager (ATM).
- Establishment to institute corrective action. Production of further product from the offending line may continue after corrective action to address the POE defects has been instigated. A new, 10 unit inspection from that line will need to be conducted during that shift. Fee-for-service charges may apply.
- Arrange to do a reinspection of the offending line produced earlier in that shift. It may be possible to inspect the product before it goes into the blast freezer or chiller. Alternatively, it can be reinspected after refrigeration. If frozen, the product for reinspection will need to be thawed.
- Record findings in Form 2 below (Appendix 2).
Instructions for completing Form 2
This form is used only after the daily product verification has identified a POE defect and a reinspection of the offending product line is triggered or for reinspection of a line of product previously designated by the packer as non-USA eligible. This was formerly referred to as a “defrost” but the product line from earlier in that shift’s production may or may not be frozen when the reinspection is conducted. An establishment supervisor must be present during the reinspection. Examine the selected product (thawed if necessary) and record only POE defects in Form 2.
Record the Product Description and the Packing Date of the offending line being reinspected.
Carton Meat
Use Table 1 below to ascertain how many cartons from the offending line must be reinspected. For instance, if when the POE defect was identified, there had been 3000kg of that line of product packed during the shift, six cartons would be selected by the department. From each of the six cartons, 5.5kg from each carton would be selected. For product lines that are primals, select sufficient pieces to make up at least 5.5kg. For instance, if the cuts weigh 2kg each, select three cuts from each carton. Don’t cut larger pieces into smaller ones to get the exact amount.
Carcases
Use Table 1 below to ascertain how many carcases to be reinspected from earlier in the shift’s production in which a POE defect was found in carcase product. For instance, if 5000kg of carcases were produced, 9 carcases would be selected by the department.
| Plan No. | Lot size | No. of sample units (Carcases/cartons) |
|---|---|---|
| 1 | Less than 3628 kg | 6 |
| 2 | 3628 kg to less than 10884 kg | 9 |
| 3 | 10884 kg to less than 27210 kg | 15 |
| 4 | 27210 kg to less than 108840 kg | 22 |
| 5 | 108840 kg to less than 226750 kg | 27 |
POE Defects
In a “defrost” reinspection, the same defect criteria are used as for the daily product verification. Do not use the criteria contained in Order 285 of the MHA Guidelines.
If any POE defects are identified during the reinspection, place a cross in the corresponding box. The offending piece of meat/carcase must be shown to the establishment representative and a copy of this form provided to them. Place the offending line of product under the department retention.
The ATM must be advised of the results of the reinspection.
If no POE Defects are found, the product line is released for all appropriate markets and for the next five shifts, the number of units for daily product verification will be temporarily increased to 15 units per shift before reverting to normal (10 units).
This form should be filed for audit purposes, regardless of whether any POE defects were identified.
Inspection of product previously declared by management to be non USA eligible
If the owner so wishes, it is possible to inspect product lines that were previously declared as non-USA eligible. The OPV/FSMA will examine the quantity of product using Table 1. If the lot is sufficient in size to fill a container, 27 cartons/carcases will be selected. If the container lot consists of product from more than one establishment, the product from each establishment will be examined separately, again using Table 1 above to ascertain the correct quantities. This reinspection may occur at a cold store or other registered establishment. Fee-for-service charges will apply.
Control of USA and non-USA eligible product
To be eligible for export to the USA, product must be nominated to the OPV/FSMA by the occupier on the day of inspection as being USA eligible. Not all USA eligible lines will be inspected necessarily and if not inspected, it does not affect the eligibility. Product nominated by the processor as not destined for the USA is not eligible to be exported to the USA. A USA-listed establishment may elect to designate all product as USA eligible, in which case no segregation is required and the department daily product hygiene verification sample can be selected from any carcase or carton product.
Occupiers must have in place systems to ensure that only product that was nominated as USA eligible on the day of inspection can be sent to the USA, unless sampled by the department at a later time. The Meat Transfer Certificates for ineligible product must not state USA eligibility or alternatively be endorsed “Not eligible for the USA unless subject to department daily product hygiene verification”. The Approved Arrangement must be amended to describe this system of control and how the department is notified of the product’s intended eligibility prior to sampling.
A load of product that had previously been identified as not eligible for the USA unless subject to the department daily product hygiene verification, may be exported to the USA if it is subject to sampling by the department as per the instructions in this notice. From Table 1, 27 units will be inspected per container.
Critical Incident Response Protocol
Level 1 Response
This level of response applies where a company has a first, finished product MHA re-inspection failure or POE rejection in the USA for port of entry defect within a 12 month window.
- Within 48 hours, the company will review its production process to determine the extent of the problem (i.e. is it a one off event or part of a production system failure). The company will make their findings available to the department.
- The company report should include:
- corrective, preventive and verification actions taken as a result of the finding,
- an assessment by the company of the risk of rejection of product from the same production system (i.e. production system failure), through an analysis of QA and production records. Defrost inspections of product types may assist companies in determining this risk.
- If the rejection is part of a production system failure the company will need to consider the status of product in store and in transit.
- The company should notify the department of any product that is at risk of rejection due to a production system failure that cannot be withdrawn from entering the market.
- The company will undertake a reassessment of the HACCP program to ensure it adequately covers the hazard of POE defects in the finished product.
- The Corrective Action Plan (CAP) will need to be developed by the company and a draft provided to the department within 10 working days.
- The company report should include:
- The department will conduct a CIR audit of the establishment’s operations.
- The audit will consist of the Field Operations Manager (FOM) and the ATM.
- The audit will cover both the company and the department on plant systems and responses.
- Should the outcome of the audit identify a production system failure, the department will consider the status of product in store and in transit.
- In determining the status of product in store and in transit:
- an analysis of the production and QA record may reveal higher risk product types
- further defrost re-inspections of higher risk product types may also be useful in determining whether or not various product types can be certified.
- The department may require additional official verification activity to ensure that compliance can be sustained.
- The additional verification will be developed to meet the particular circumstances but will most likely be maintained for a minimum of 4 weeks.
Level 2 Response
This level of response applies where a company has received a second MHA re-inspection failure for POE defects or POE rejection in the USA within a 12 month window or has not developed an adequate response to a show cause letter or failed a department critical incident response audit in response to a Level 1 detection.
The response from both the company and the department will require at least the same response as for a Level 1 Response above but will include the additional responses listed below.
- The department will, after the second MHA re-inspection failure or POE detection, issue a formal ‘show cause’ letter asking the company to explain why they should not be delisted for the market.
- The company response will need to give the department the confidence that they can continue to certify product for that market.
- In addition to the response in the level 1 response, a CAP will need to be developed by the company and a draft provided to the department within 10 working days.
- The department may require additional verifications by the company to provide the necessary integrity to certification.
- The department critical incident response audit will be conducted by an independent expert team.
Level 3 Response
This level of response applies where a company has received a third MHA re-inspection failure or POE rejection in the USA for POE defects within a 12 month window or has not developed an adequate response to a show cause letter or failed a department critical incident response audit in response to a Level 1 or 2 detection.
- The department will not provide certification for the species concerned.
- In addition to the response in the level 1 response, a CAP will need to be developed by the company and a draft provided to the department within 10 working days.
- The department will conduct a critical incident response audit by an independent team.
- Access to other markets will be reviewed to ensure compliance with their requirements.
- To regain market access the processor must be able to effectively implement their CAP and demonstrate sustained compliance for a period of not less than 4 weeks.
Appeals Mechanism
If more than one rejection has been notified by the USA authorities, the department will examine the circumstances relating to those rejections and decide which level of response is the most appropriate.
In the past, multiple rejections may have occurred due to faulty product that was produced in the same time period but shipped in different containers. In their response to the show cause letter, the establishment has the opportunity to explain to the department why they believe the particular critical response level should be amended.
In consideration of that request, the department will consider each situation on its merits, being guided by market expectations and the risk of further violations. Consideration will be given to the amount of product successfully exported by the establishment to that market and the proportion of rejected product. This data should be included in the response to the show cause letter if the establishment wishes to do so. The delegation for the final decision will reside with the Assistant Secretary.
Responsibilities
Department on-plant staff
- Daily examine 10 units of sheep, lamb and goat carcases and carton meat that the company has identified as being USA eligible. This will replace the twice weekly verification of the boning room pre-trim MHA previously required.
- In the event of a finding of POE defects on the finished product, conduct a “defrost” re-inspection using MHA procedures.
Establishment management
- The occupier will inform the OPV/FSMA on a daily basis what products are USA eligible.
- Conduct MHA and contribute the data to the PHI as per Meat Notice 2013/01.
- Respond to the department MHA verification findings as per Meat Notice 2013/01.
- Apply corrective and preventive action as necessary.
ATM
- Along with the FOM, will conduct CIR audits in the event that the defrost re-inspection “fails”.
FOM
- Conduct CIR audits.
- Determine the status of product in store and in transit as part of the CIR audit.
Records
The establishment must maintain all production records for two years.
References
- Export Control (Meat and Meat Products) Orders 2005
- FSIS PHIS Directive 9900.2 Reinspection of meat, poultry, and egg products.
- Market Access Advice 1232 USA – Port of Entry Detections
- Meat Hygiene Assessment – 2nd edition
- Meat Notice 2013/01
Samantha Allan
National Veterinary Technical Manager
Export Meat Program
