This indicator assesses the risks to loss of forest genetic variation and describes the formal measures designed to mitigate this risk. A loss of genetic diversity in species can result in a decreased ability to adapt to future environmental change, and thus a higher risk of extinction.
This is Key information for Indicator 1.3a, published October 2024.
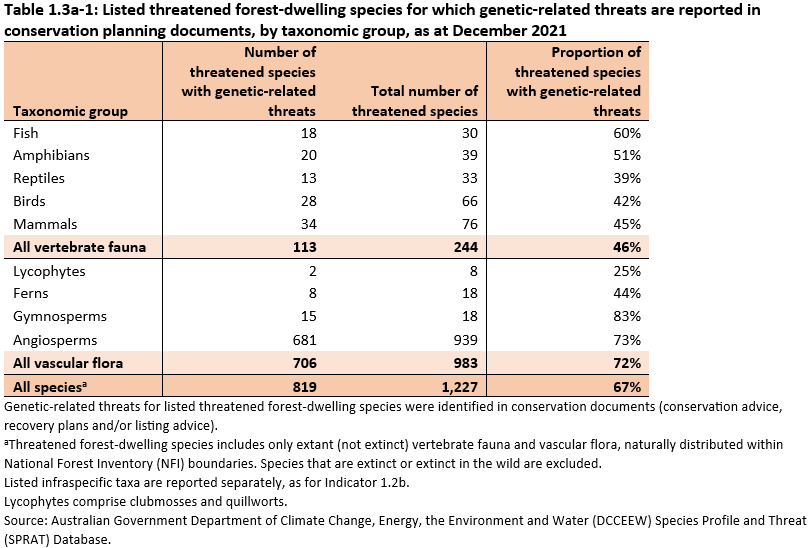
- Genetic-related threats were identified for 819 (67%) of the 1,227 listed threatened forest-dwelling species (as at December 2021), specifically:
- 113 of 244 (46%) of threatened forest-dwelling vertebrate fauna species
- 706 of 983 (72%) of forest-dwelling threatened vascular flora species.
- The most common genetic-related threats for both threatened forest-dwelling fauna and flora were small populations, fecundity issues, and fragmented populations.
- Australia relies on in situ conservation of forest genetic resources as the main mechanism for conservation of forest genetic resources.
This indicator focuses on genetic-related threats for threatened forest-dwelling species. All 1,277 threatened forest-dwelling species are assessed against specific genetic-related threat categories: small populations, fragmented populations, low genetic diversity, hybridisation and fecundity issues.
Genetic variation is an essential component of the diversity of Australia’s forests and forest species, and their productive capacity. Understanding Australia’s forest genetics allows better conservation management of forests and forest species, and better management and development of forest resources (Lott and Read 2021).
The number of forest-dwelling native fauna and flora for which data on genetic diversity are available remains small, although knowledge in this field is increasing. Information on the structure of species genetic diversity is increasingly being used to underpin biodiversity management, including conservation efforts after disturbance events (Catullo and Moritz 2021). The overall status of Australia’s forest genetic resources, and implementation of genetic resource conservation mechanisms, are described in Indicator 1.3b.
Key drivers of risks to forest-dwelling species genetic variation include forest fragmentation, small populations and climate change. These factors can expose species to increased extinction risks due to loss of genetic variation. Species with a lower level of genetic variation are less able to respond to gradual or immediate threats, or adapt to change, and so face a higher risk of extinction (Saunders et al. 1998), although other factors can apply for individual species. While it is difficult to determine how much of the genetic variation within a species has been historically lost, it is possible to identify if certain species are becoming threatened by the increased isolation of specific populations due to habitat depletion and fragmentation, and by other threatening abiotic and biotic factors such as those discussed in Indicator 1.2b. Genetic-related threats are increasingly being addressed in the conservation planning documents (conservation advice, recovery plans and/or listing advice) for threatened species.
The process of forest fragmentation (see Indicator 1.1d), mainly caused by clearing for agriculture and urban and industrial development, is a significant contributor to a reduction in genetic variation of certain species. Populations that have become fragmented are at greater risk of restricted gene flow between populations, potential inbreeding and progressive loss of genetic diversity. Populations at greatest risk and of greatest concern are those that are already small or fragmented and with high conservation value.
Climate change is likely to have complex and far-reaching effects on population genetics, influencing connectivity, diversity, adaptation, and overall evolution of species. It is expected to lead to losses in genetic connectivity between populations as climatic zones shift, particularly for species with naturally restricted geographic ranges and habitats, or narrow climate niches (Hoffmann et al. 2019; Meza-Joya et al. 2023). Climate change can also disrupt the interaction of plants with pollinators, potentially reducing pollination with consequences for increased inbreeding and losses of genetic variation.
Changes in fire regimes and fire-related ecological processes can drive declines in genetic diversity (DAWE 2022). Changes in fire regimes are linked to both changes in human interaction with fire in the landscape since European colonisation of Australia and with climate change. The lack of fire, and more frequent high severity fires, can disturb reproduction and recruitment processes, reduce population size and consequently lower genetic variation.
Genetic-related threats are identified for 819 (67%) of the 1,227 threatened forest-dwelling species listed as at December 2021 (Table 1.3a-1). Genetic-related threats in the context of this Indicator are small populations, fragmented populations, low genetic diversity, hybridisation, and fecundity issues (see Supporting Information for Indicator 1.3a for definitions).
Genetic-related threats were more commonly cited for flora than for fauna, and were specified for:
- 113 of 244 species of forest-dwelling threatened vertebrate fauna (46%)
- 706 of 983 species of forest-dwelling threatened vascular flora (72%).
The 113 fauna species with genetic-related threats are represented almost equally across the five taxon groups (Table 1.3a-1). Fish have the largest proportion of species with genetic-related threats (60%), and reptiles have the lowest proportion (42%).
A total of 681 angiosperm species (flowering plants) have genetic-related threats (Table 1.3a-1), which is 73% of the threatened forest-dwelling angiosperms. This includes:
- 80% of 185 threatened forest-dwelling Orchidaceae
- 70% of 128 threatened forest-dwelling Myrtaceae
- 74% of 93 threatened forest-dwelling Fabaceae
- 84% of 83 threatened forest-dwelling Proteaceae
Threatened angiosperms with genetic-related threats include species of high economic importance, such as Queensland nut tree (Macadamia integrifolia) and rough-shelled bush nut (M. tetraphylla). The other macadamia species less frequently used in commercial production, gympie nut (M. ternifolia) and bulburin nut tree (M. jansenii), are also threatened forest-dwelling species and identified to be prone to genetic-related threats.
Fifteen gymnosperm species (83% of the threatened forest-dwelling gymnosperms) have genetic-related threats (Table 1.3a-1). Most are cycads from the families Cycas and Macrozamia, but also include wollemi pine (Wollemii nobilis).
Click here for a Microsoft Excel workbook of the data for Table 1.3a-1.
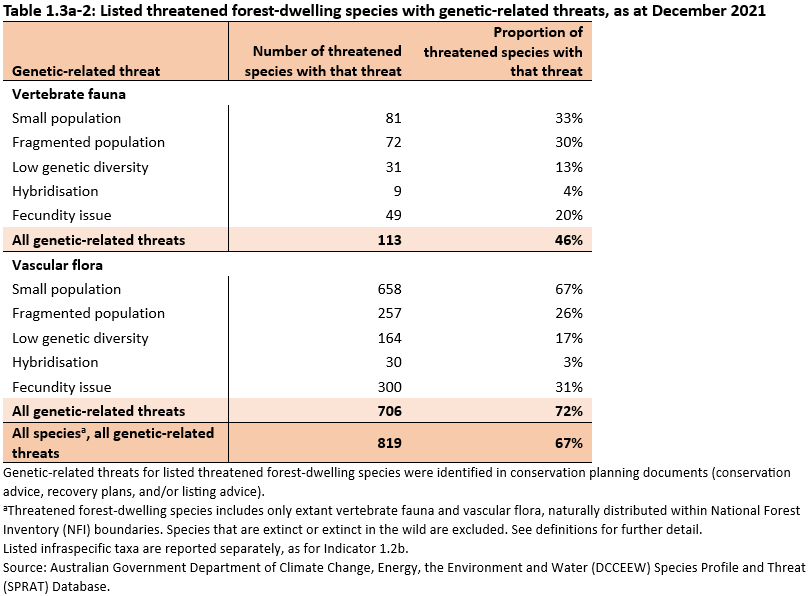
Genetic-related threats often operate simultaneously to drive species extinction. The most common genetic-related threats for threatened forest-dwelling species were (Table 1.3a-2):
- small population (33% and 67% of total threatened forest-dwelling fauna and flora and, respectively)
- fecundity issues (20% and 31% of total threatened forest-dwelling fauna and flora, respectively)
- fragmented population (30% and 26% of total threatened forest-dwelling fauna and flora, respectively).
There was a higher proportion of threatened forest-dwelling flora species with extremely small population size (consisting of fewer than 1,000 individuals) than forest-dwelling fauna. Several flora species are known to only have fewer than 10 individuals remaining in the wild, for example, mongarlowe mallee (Eucalyptus recurva), slender-nerved acacia (Acacia leptoneura), dwarf-spider orchid (Caladenia pumila), Trigwell’s rullingia (Commersonia erythrogyna) and Bolivia Hill rice-flower (Pimelea venosa).
Hybridisation is specified as a genetic risk to only 4% of threatened forest-dwelling fauna and 3% of threatened forest-dwelling flora, but when it occurs, can lead to a rapid decline in the numbers of genetically pure individuals.
Click here for a Microsoft Excel workbook of the data for Table 1.3a-2.
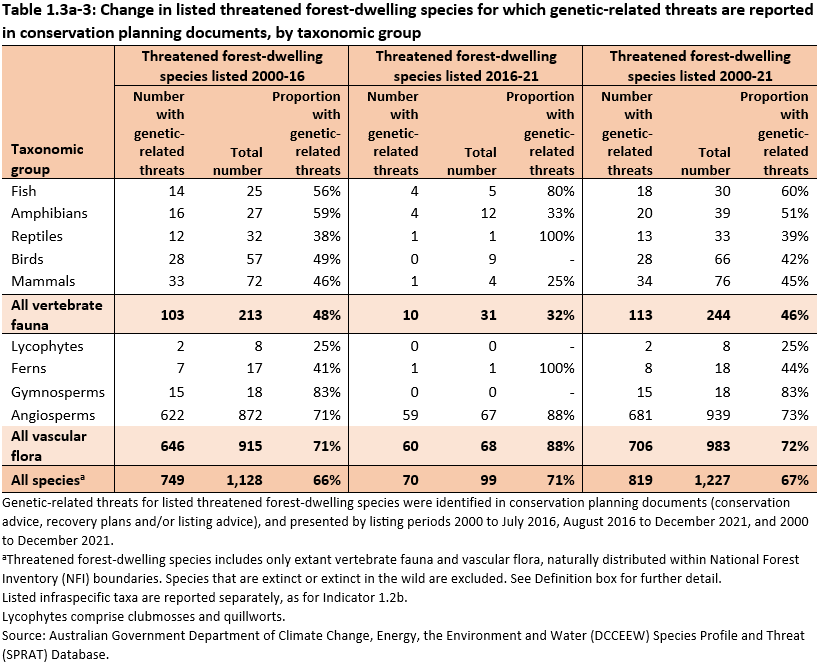
The number of threatened forest-dwelling flora and fauna identified with genetic-related threats have changed over time (Table 1.3a-3):
- 71% (70 of the 99) of threatened forest-dwelling flora and fauna species listed between August 2016 and December 2021 have genetic-related threats, compared to 66% (749 of 1,128 species) listed between 2000 and August 2016
- a greater proportion (88%) of flora listed between August 2016 and December 2021 (60 of 68 species) have genetic-related threats compared to 71% of flora listed between 2000 and August 2016 (646 of 915 species)
- a smaller proportion (32%) of fauna listed between August 2016 and December 2021 (10 of 31 species) have genetic-related threats compared to 48% of fauna listed prior to August 2016 (103 of 213 species).
Click here for a Microsoft Excel workbook of the data for Table 1.3a-3.
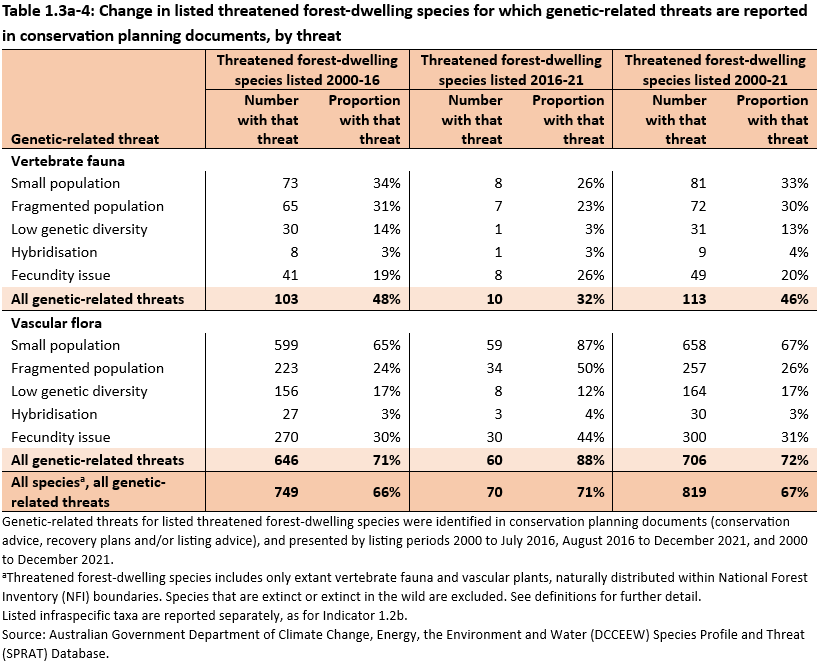
Small population, fragmentation and fecundity are the most common genetic-related threats for listed threatened forest-dwelling species over time (Table 1.3a-4). Small population and fragmentation are increasingly recognised in recent listings for flora. Fecundity issues are increasingly recognised in recent listings for both flora and fauna. The increased recognition of genetic risks in conservation planning documents are largely due to the growing body of knowledge on the species genetics structure and population quantitative genetics, as well as aggregating observed evidence on increased extinction risk from having little genetic exchange (fragmented and isolated population), inbreeding (small population), or other genetic consequences from various threats.
Click here for a Microsoft Excel workbook of the data for Table 1.3a-4.
Australia relies on in situ conservation of forest genetic resources as the main mechanism for conservation of forest genetic resources. In situ conservation is the conservation of species and genetic components of biological diversity in their natural habitats. All 115 native species and hybrids listed as forest genetic resources for Australia by the United Nations Food and Agriculture Organization (FAO) have populations conserved in situ through formal and informal reserved and protected areas (Lott and Read 2021; Indicator 1.3b). The area of forest reserved and managed for protection through formal and informal processes is described in Indicator 1.1c.
In situ recovery actions for listed threatened species can include habitat restoration, wildlife corridors, engineered animal movement mechanisms (e.g. possum bridges), seed-collecting programs (for subsequent resowing), and management of habitat and populations under forest management systems (e.g. forest management plans and code of practice systems that include prescription on fire management, feral animal and weed management, selection of genetic resources (provenances) for planting, etc.).
Ex situ conservation is the conservation of species and genetic components of biological diversity outside their natural habitats. Ex situ conservation for flora in Australia occurs through botanic gardens, seed banks, provenance or clonal plantings, seed orchards, and seed production areas to complement in situ conservation. Ex situ seed banking is considered a vital element of the conservation of Australia’s flora, with 67% of listed threatened flora species represented in conservation seed banks (Martyn Yenson et al. 2024). The main conservation seed banks for native species, including threatened forest-dwelling species, are managed by the Australian Seed Bank Partnership (Martyn Yenson et al. 2021). The National Macadamia Germplasm Collection is an example of ex situ conservation plantings and breeding trials for threatened species. Other seed orchards and conservation plantings for threatened forest-dwelling flora species are listed in Lott and Read (2021).
Ex situ conservation for fauna in Australia is supported through establishing populations in zoos and aquariums, captive breeding and translocation programs. Newhaven Wildlife Sanctuary in Northern Territory, that is Australia’s largest fenced feral cat and fox free area, is an example of ex situ conservation for threatened fauna. This sanctuary supports conservation of forest-dwelling fauna such as mala (Lagorchestes hirsutus), red-tailed phascogale (Phascogale calura), western quoll (Dasyurus geoffroii) and golden bandicoot (Isoodon auratus).
Ex situ conservation supports population recovery in situ, largely through translocations. Translocation is the establishment and augmentation of populations using individuals (genetic materials) that have been produced ex situ. Translocations are being increasingly proposed as a way of conserving biodiversity, particularly for threatened and keystone species (Weeks et al. 2011, Zimmer et al. 2019). A well designed and managed translocation that places a strong focus on restoring genetic variability is imperative to ensure the long-term persistence of translocated populations. A step-by-step best-practice guide for conservation translocation of Australian flora is available from the Australian Network for Plant Conservation Guidelines for the translocation of threatened plants in Australia (Commander et al. 2018).
Genetic studies to determine effective population size and within- and among-population genetic diversity, and restoring population connectivity, are examples of key recovery actions specified in conservation documents for EPBC-listed species with genetic-related risks.
Catullo R, Moritz C (2021). Genetic assessment of bushfire-impacted vertebrate species. Final Report. Threatened Species Recovery Hub, National Environmental Science Programme.
Commander LE, Coates D, Broadhurst L, Offord CA, Makinson RO, Matthes M (2018). Guidelines for the translocation of threatened plants in Australia, Third Edition, Australian Network for Plant Conservation, Canberra.
DAWE (Department of Agriculture, Water and the Environment) (2022). Fire regimes that cause declines in biodiversity as a key threatening process, Australian Government, Canberra, April 2022. CC BY 4.0.
Hoffmann AA, Rymer PD, Byrne M, Ruthrof KX, Whinam J, McGeogh M, Bergstrom DM, Guerin GR, Sparrow B, Joseph L, Hill SJ, Andrew NR, Camac J, Bell N, Riegler M, Gardner JL, Williams SE (2019). Impact of recent climate change on terrestrial flora and fauna: Some emerging Australian examples, Austral Ecology 44:3-27. doi.org/10.1111/aec.12674
Lott R, Read SM (2021). Status of Australia’s Forest Genetic Resources 2021. Australia’s Country Report for The Second Report on the State of the World’s Forest Genetic Resources, Prepared for the Food and Agriculture Organization of the United Nations. ABARES Research Report 21.15. November 2021, Canberra. CC BY 4.0. doi.org/10.25814/dnv3-vj64
Martyn Yenson AJ, Offord CA, Meagher PF, Auld T, Bush D, Coates DJ, Commander LE, Guja LK, Norton SL, Makinson RO, Stanley R, Walsh N, Wrigley D, Broadhurst L (2021). Plant Germplasm Conservation in Australia: strategies and guidelines for developing, managing and utilising ex situ collections, Third Edition. Australian Network for Plant Conservation, Canberra.
Martyn Yenson AJ, Sommerville KD, Guja LK, Merritt DJ, Dalziell EL, Auld TD, Broadhurst L, Coates DJ, Commander L, Crawford AD, Emery NJ, Funnekotter B, Knapp Z, Makinson RO, Monks L, Wrigley D, Offord CA (2024). Ex situ germplasm collections of exceptional species are a vital part of the conservation of Australia’s national plant treasures, Plants, People, Planet 6(1):44-66. doi.org/10.1002/ppp3.10421
Meza-Joya FL, Morgan-Richards M, Koot EM, Trewick SA (2023). Global warming leads to habitat loss and genetic erosion of alpine biodiversity. Journal of Biogeography 50, 961–975. doi.org/10.1111/jbi.14590
Saunders D, Margules C, Hill B (1998). Environmental indicators for national state of the environment reporting: biodiversity, Australia: State of the Environment (Environmental Indicator Reports), Department of the Environment, Canberra.
Weeks AR, Sgro CM, Young AG, Frankham R, Mitchell NJ, Miller KA, Byrne M, Coates DJ, Eldridge MDB, Sunnucks P, Breed MF, James EA, Hoffmann AA (2011). Assessing the benefits and risks of translocations in changing environments: a genetic perspective, Evolutionary Applications 4(6):709-725. doi.org/10.1111/j.1752-4571.2011.00192.x
Zimmer HC, Auld TD, Cuneo P, Offord CA, Commander LE (2019). Conservation translocation – an increasingly viable option for managing threatened plant species, Australian Journal of Botany 67: 501-509. doi.org/10.1071/BT19083
Further information
- Assessment of genetic-related threats
- Genetic-related threats in different listing periods